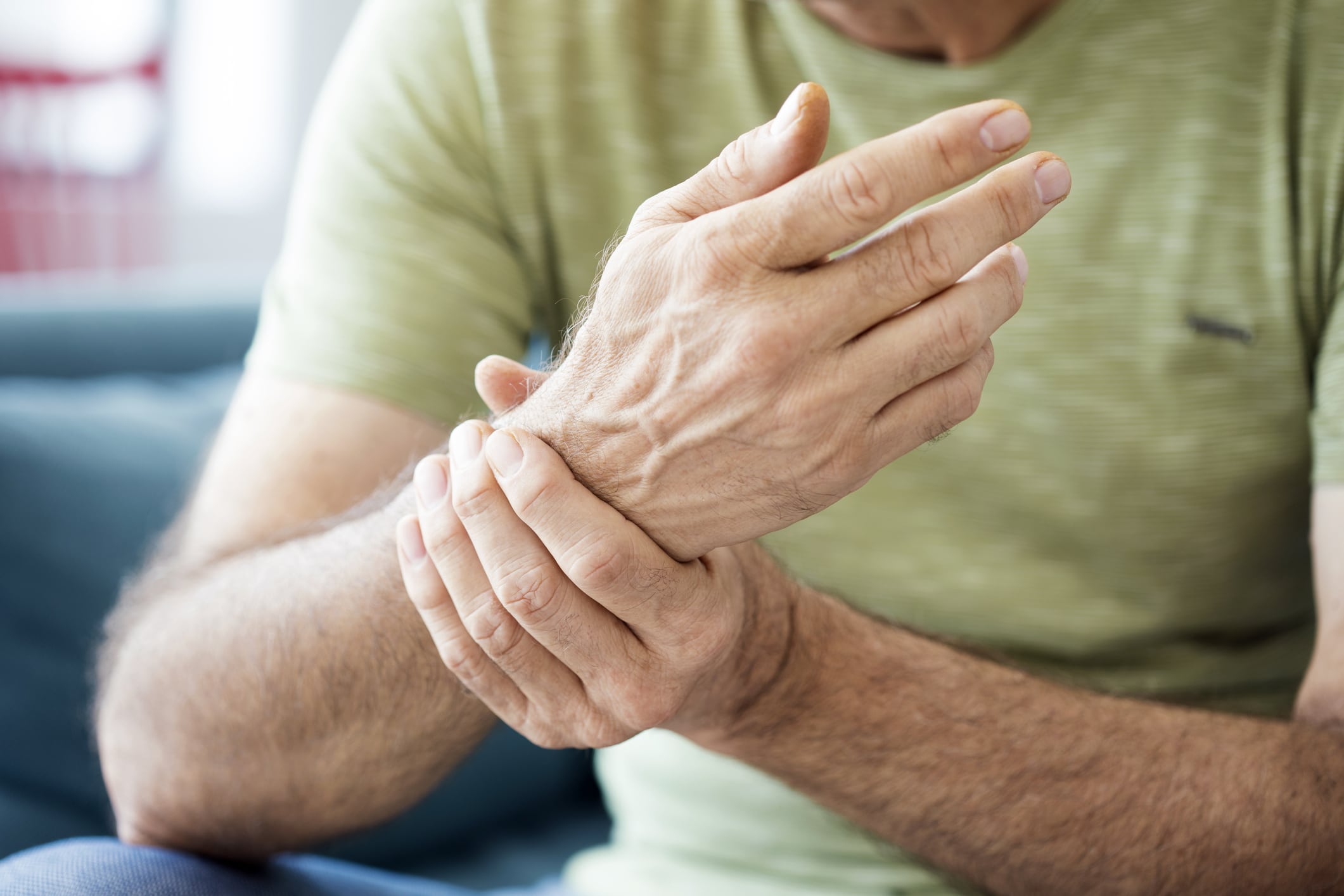
- Gout is a common type of inflammatory arthritis that can be very painful(1). Repeated gout flares can result in gouty arthritis, a worse kind of arthritis.
- CBD (cannabidiol) contains anti-inflammatory properties that may help with pain caused by arthritis(2).
- Gout results from excess uric acid crystals in the body(3). Studies have discussed that short sleep duration is linked to high uric acid levels(4).
- Research from Harvard Medical School stated that CBD may reduce symptoms of anxiety and, consequently, help individuals get quality sleep(5).
Does CBD Help With Gout?
Currently, there is no proof that CBD may work on gout. However, research on the anti-inflammatory properties of CBD showed that the cannabinoid may reduce pain and inflammation among individuals, such as those who experience arthritis pain(6).
According to experts, the benefits of CBD include controlling symptoms of chronic pain(7), such as arthritis caused by swelling of the joints(8).
Both arthritis and gout result in intense pain. Individuals who experience this may take pain medication to reduce the effects of gout(9).
However, studies suggested that CBD’s therapeutic effects may work for anti-inflammatory purposes. CBD may react with the body’s endocannabinoid system, which may work with the nervous system to control inflammation(10).
The endocannabinoid system, or ECS, is a vast network of neurons responsible for regulating other biological systems in the body, such as the immune system(11).
The ECS also maintains homeostasis, the state in which the body maintains a balanced internal environment amidst circumstances, primarily physical, psychological, and emotional stimuli(12).
The ECS comprises endogenous cannabinoids, which are cannabinoids naturally produced by the human body. Their primary function is signaling the nervous system to attend to body pain through receptors(13).
The ECS also consists of cannabinoid receptors called the CB1 and CB2 receptors(14).
CB1 receptors usually deal with body pain, anxiety, and mood regulation. In contrast, CB2 receptors respond to inflammation, mental health issues such as depression, post-traumatic stress disorder, and autoimmune diseases(15).
When an individual takes CBD, the cannabinoid receptors respond by regulating pain and inflammation in the body(16). Thus, taking CBD products may indirectly reduce symptoms of gout.
However, as there is a lack of evidence on the efficacy of CBD as an alternative treatment, it is best to consult with healthcare professionals to know the best medication for this condition.
Research and Studies on CBD for Gout
No research or study has concluded that CBD works as an effective cure for gout. However, some of CBD’s characteristics may indirectly help with this condition.
Excess uric acid crystals in the body can lead to gout(17). Studies have shown that short sleep duration may lead to high uric acid levels(18).
Meanwhile, research from Harvard Medical School in 2021 showed that CBD may alleviate symptoms of various medical conditions and help individuals sleep better(19).
The 2021 research also showed that CBD may reduce fibromyalgia symptoms, a condition that causes vast pain among individuals(20). Severe joint pain is one of the symptoms of gout(21).
Although the cause of fibromyalgia is untraceable, the disease may occur among individuals with depression, anxiety, poor digestion, and rheumatoid issues, like arthritis(22).
If the cause of joint pain in an individual is rooted in large amounts of uric acid in the body, the pain may count as a severe case of arthritis called gout(23).
Nonetheless, individuals should seek assistance from a healthcare professional before choosing the appropriate treatment for gout.
Is CBD Safe for Gout?
There is no CBD product for gout approved by the Food and Drug Administration (FDA). Moreover, CBD has exhibited side effects among individuals taking other medications(24).
If an individual is already taking pain medication for gout, the addition of CBD may cause health issues, such as liver damage(25).
However, studies suggest that taking low doses of CBD alone may help reduce pain and inflammation altogether(26).
To avoid risks and side effects, individuals should consult their respective healthcare providers for more information on the pharmacology of CBD.
How to Take CBD for Gout
CBD for gout may come in several forms. Individuals may choose which CBD product to use for body pain and sleep problems caused by gout.
Oral administration, vaping, and topical application using CBD lotions may be suitable delivery methods to take CBD. However, individuals may also opt for CBD tinctures and edible CBD products such as gummies to help manage body pain.
Another way for individuals to determine the best CBD product for gout is to know the different types of CBD, namely full-spectrum, broad-spectrum, and isolate CBD.
Full-spectrum CBD oil contains cannabinoids and phytochemicals (plant-based chemicals), like terpenes and flavonoids, and less than 0.3% THC (tetrahydrocannabinol) concentration(27).
Terpenes are essential oil constituents that give cannabis its distinctive aroma(28). Meanwhile, flavonoids are plant compounds with antimicrobial and antioxidant benefits(29).
Broad-spectrum CBD oil is mostly similar to full-spectrum CBD oil. However, the THC content in broad-spectrum CBD is little to none(30).
On the other hand, isolate CBD consists of pure CBD and no THC(31).
How Does CBD Oil Work to Help Alleviate Gout Symptoms?
Individuals, especially those experiencing pain from inflammation, may use CBD oil for overall wellness.
According to studies, CBD use may help decrease inflammation by interacting with the body’s ECS(32). When an individual takes CBD, the cannabinoid sends signals to the brain through the ECS and regulates the body’s immune response.
A 2020 study published in the journal Current Pharmaceutical Biotechnology showed that individuals may experience relief after using CBD oil for neuropathic pain(33).
Another study showed the effectiveness of CBD on osteoarthritis in animals, suggesting a similar potential application in humans(34).
However, existing studies that show the positive effects of CBD on gout symptoms are limited.
The Pros and Cons of CBD Oil for Gout
The pros and cons of taking CBD oil for gout may depend on an individual’s current medication and the severity of gout pain.
Individuals with gout may change their lifestyle, including stopping the intake of certain medications and food(35). In addition, these individuals may also use CBD oil or topical CBD to supplement treatment for joint pain(36).
As with any medication, cannabis products may develop into tolerance, wherein the liver processes drugs more quickly than usual(37). The risk of tolerance in CBD use may reduce the potency of the cannabinoid.
Furthermore, using CBD oil with other medications may cause long-term side effects, which experts are yet to explore(38).
What Is the Suitable CBD Dosage for Gout?
The FDA has not authorized CBD use for the treatment of diseases. Additionally, sources that show the benefits of CBD are limited.
Therefore, there is no suitable dosage for CBD in the effective treatment of gout.
However, studies showed a decrease in inflammation among individuals using CBD. According to one review, CBD or a combination of CBD and THC may exert a predominantly anti-inflammatory effect(39).
Individuals who wish to find a suitable treatment for gout symptoms may speak to a professional knowledgeable in the condition. Consumers using CBD for the first time must seek medical consultation to minimize or avoid CBD’s side effects.
Is CBD Legal?
Recently, the FDA has shown interest in the benefits of CBD as this compound has become popular among consumers in the U.S. However, the agency is concerned about how companies market CBD products.
Researchers observed that certain products are incorrectly labeled. For example, some CBD products contain high amounts of THC, and individuals may take this psychoactive compound without their knowledge(40).
The FDA has approved only one CBD drug, called Epidiolex, which may treat certain types of epilepsy(41).
In order to learn more about the legality of CBD or medical cannabis in the U.S., individuals must review their state’s laws, typically accessible on U.S. state government websites.
What Is Gout?
Gout is a form of arthritis caused by uric acid buildup in the affected joints. Gout occurs when the body digests purine-rich foods, such as red meat(42).
A severe type of arthritis, gout pain appears in the big toe and may show no signs of distress. When this sign appears, experts call it remission(43).
Individuals may also experience gout flare-ups when gout worsens. If left untreated, the condition may develop into gouty arthritis, a more severe case of arthritis(44).
There is currently no over-the-counter medication available for the treatment of gout. However, individuals may take analgesic medicine for pain relief, like ibuprofen and other NSAIDs.
What Are the Most Common Triggers of Gout?
Individuals who frequently eat purine-rich foods, such as liver and other organ meat, are prone to gout(45). High-fructose foods may also trigger the disease(46).
Additionally, the rising prevalence of gout may be related to the prevalence of conditions associated with diabetes, hypertension, and obesity(47).
What Are the Symptoms of Gout?
Individuals with gout may experience severe pain and flare-ups, and joint pain in the body’s lower regions, like the knees, toes, and ankles.
Other symptoms of gout include swelling and redness around the joints(48).
What Are the Risk Factors for Gout?
Individuals may have a higher risk of gout when diagnosed with obesity, often rooted in an individual’s family history(49).
Gout attacks may also be more common among males due to their bodies’ naturally higher uric acid levels(50). Studies have shown that short sleep duration is linked to high uric acid levels.
Individuals with the following medical conditions may also develop gout and gout arthritis:
- High blood pressure
- Heart failure
- Diabetes or insulin resistance
- Kidney issues
How Can Individuals Prevent Gout?
Some individuals may develop gout due to their existing biological makeup. To avoid worsening its symptoms, individuals may change their diet by avoiding certain foods that trigger gout and taking antioxidants, such as essential oils(51).
As gout has no proper cure, individuals may instead take medications that help reduce the symptoms of the disease.
The following anti-inflammatory drugs may help alleviate the symptoms of gout(52):
- Ibuprofen
- Naproxen
- Corticosteroids
Alternative Treatments for Gout
Individuals with gout may resort to lifestyle changes, including changes in diet. To reduce gout symptoms, individuals should consider avoiding fructose and purine-rich foods(53).
One study postulated that medical marijuana may lower blood pressure levels among adults(54). Hypertension, or high blood pressure, may cause gout in some individuals(55).
CBD is marijuana’s second most prevalent active ingredient, next to THC(56). However, medical marijuana is not FDA-approved.
Before taking any alternative medicine, individuals must first consult a doctor for suitable treatment of inflammatory diseases like gout.
Can Individuals Use Essential Oils for Gout?
A study showed that essential oils extracted from lemongrass may decrease uric acid levels among older women(57).
Another study that used a rat model of arthritis to test the efficacy of essential oils from a species of cinnamon leaves also showed positive results(58).
These studies suggest that essential oils may have the potential to help control the symptoms of gout.
- Gout.
https://www.cdc.gov/arthritis/basics/gout.html - Antioxidative and Anti-Inflammatory Properties of Cannabidiol. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7023045/
- Gout.
https://my.clevelandclinic.org/health/diseases/4755-gout - Association of sleep quality and sleep duration with serum uric acid levels in adults.
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0239185 - CBD products are everywhere. But do they work? https://www.health.harvard.edu/newsletter_article/cbd-products-are-everywhere-but-do-they-work
- Antioxidative and Anti-Inflammatory Properties of Cannabidiol. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7023045/
- Cannabidiol (CBD)-what we know and what we don’t. https://www.health.harvard.edu/blog/cannabidiol-cbd-what-we-know-and-what-we-dont-2018082414476
- Arthritis. https://www.mayoclinic.org/diseases-conditions/arthritis/symptoms-causes/syc-20350772
- Gout.
https://www.cdc.gov/arthritis/basics/gout.html - Cannabidiol.
https://pubchem.ncbi.nlm.nih.gov/compound/Cannabidiol - An Introduction to the Endogenous Cannabinoid System. https://pubmed.ncbi.nlm.nih.gov/26698193/
- Healing with CBD, page 58. https://drive.google.com/file/d/1AGlxnhS2SoFeOXEuysv75bd_C9pEnwsU/view
- Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC5877694/
- Ibid.
- Healing with CBD, page 68. https://drive.google.com/file/d/1AGlxnhS2SoFeOXEuysv75bd_C9pEnwsU/view
- Healing with CBD, page 146. https://drive.google.com/file/d/1AGlxnhS2SoFeOXEuysv75bd_C9pEnwsU/view
- Gout.
https://my.clevelandclinic.org/health/diseases/4755-gout - Association of sleep quality and sleep duration with serum uric acid levels in adults. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0239185
- CBD products are everywhere. But do they work? https://www.health.harvard.edu/newsletter_article/cbd-products-are-everywhere-but-do-they-work
- Ibid.
- Gout: Symptoms & Causes. https://www.mayoclinic.org/diseases-conditions/gout/symptoms-causes/syc-20372897
- Fibromyalgia.
https://www.cdc.gov/arthritis/basics/fibromyalgia.htm - Gout: An old disease in new perspective – A review. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5512152/
- What are the benefits of CBD — and is it safe to use? https://www.mayoclinic.org/healthy-lifestyle/consumer-health/expert-answers/is-cbd-safe-and-effective/faq-20446700
- CBD and other medications: Proceed with caution. https://www.health.harvard.edu/blog/cbd-and-other-medications-proceed-with-caution-2021011121743
- Cannabidiol (CBD)-what we know and what we don’t. https://www.health.harvard.edu/blog/cannabidiol-cbd-what-we-know-and-what-we-dont-2018082414476
- Cannabidiol primer for healthcare professionals. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7340472/
- The Cannabis Terpenes. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7763918/
- Flavonoids as antioxidants.
https://pubmed.ncbi.nlm.nih.gov/10924197/ - Ibid.
- Ibid.
- Cannabidiol primer for healthcare professionals. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7340472/
- The Effectiveness of Topical Cannabidiol Oil in Symptomatic Relief of Peripheral Neuropathy of the Lower Extremities.
https://pubmed.ncbi.nlm.nih.gov/31793418/ - What Research Says About CBD Oil. https://www.nm.org/healthbeat/medical-advances/science-and-research/what-research-says-about-cbd-oil
- Gout.
https://www.cdc.gov/arthritis/basics/gout.html - Cannabidiol primer for healthcare professionals. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7340472/
- Healing with CBD, page 83-84. https://drive.google.com/file/d/1AGlxnhS2SoFeOXEuysv75bd_C9pEnwsU/view
- CBD Oil — Are the Benefits Claimed Too Good To Be True? https://health.clevelandclinic.org/cbd-oil-benefits/
- The Effects of Cannabinoids on Pro- and Anti-Inflammatory Cytokines: A Systematic Review of In Vivo Studies. https://www.liebertpub.com/doi/abs/10.1089/can.2020.0105
- Penn Study Shows Nearly 70 Percent of Cannabidiol Extracts Sold Online Are Mislabeled. https://www.pennmedicine.org/news/news-releases/2017/november/penn-study-shows-nearly-70-percent-of-cannabidiol-extracts-sold-online-are-mislabeled
- Better Data for a Better Understanding of the Use and Safety Profile of Cannabidiol (CBD) Products. https://www.fda.gov/news-events/fda-voices/better-data-better-understanding-use-and-safety-profile-cannabidiol-cbd-products
- Potential new target for treatment of gout. https://www.sciencedaily.com/releases/2019/10/191029095629.htm
- Gout.
https://www.cdc.gov/arthritis/basics/gout.html - Ibid.
- Ibid.
- Management of Gout. https://effectivehealthcare.ahrq.gov/products/gout/research-protocol
- Ibid.
- Gout. https://www.cdc.gov/arthritis/basics/gout.html
- Ibid.
- Gout.
https://my.clevelandclinic.org/health/diseases/4755-gout - Chemical composition and antibacterial and antioxidant properties of commercial essential oils. https://www.sciencedirect.com/science/article/abs/pii/S0926669012004475
- Management of Gout. https://effectivehealthcare.ahrq.gov/products/gout/research-protocol
- Gout. https://www.mayoclinic.org/diseases-conditions/gout/symptoms-causes/syc-20372897
- Cannabis is associated with blood pressure reduction in older adults – A 24-hours ambulatory blood pressure monitoring study. https://pubmed.ncbi.nlm.nih.gov/33483174/
- Gout.
https://my.clevelandclinic.org/health/diseases/4755-gout - PDEA Reiterates Warning On Cannabidiol (CBD) Oil. https://pdea.gov.ph/2-uncategorised/1488-pdea-reiterates-warning-on-cannabidiol-cbd-oil
- Effect of the Stems Lemongrass (Cymbopogon citratus) in Pallumara and Pepes Anchovy (Stolephorus Sp.) to Uric Acid Levels of Hyperuricemia Elderly Women. https://oamjms.eu/index.php/mjms/article/download/5203/5187/33927
- Essential oil from leaves of Cinnamomum osmophloeum acts as a xanthine oxidase inhibitor and reduces the serum uric acid levels in oxonate-induced mice. https://pubmed.ncbi.nlm.nih.gov/18693097/













